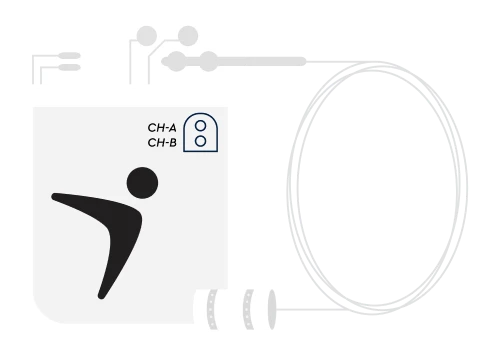
The Altius System Implantable Pulse Generator sends electrical stimulation directly to the source of the pain and is ready to activate when you need it.

Take Power Over Pain
Altius is an FDA-approved, first-of-its-kind device that gives you the power to control your chronic post-amputation pain and get relief whenever you need it.
Rise up and take down pain

POWER OVER PAIN
The Altius Direct Electrical Nerve Stimulation System is FDA-approved to help adults living with lower limb loss who have chronic post-amputation pain, such as phantom limb pain or residual limb pain. This patient-controlled, implanted electrical stimulation device gives you the power to start treatments when you need them.
On Demand
Pain Relief
Patient-controlled
and customizable
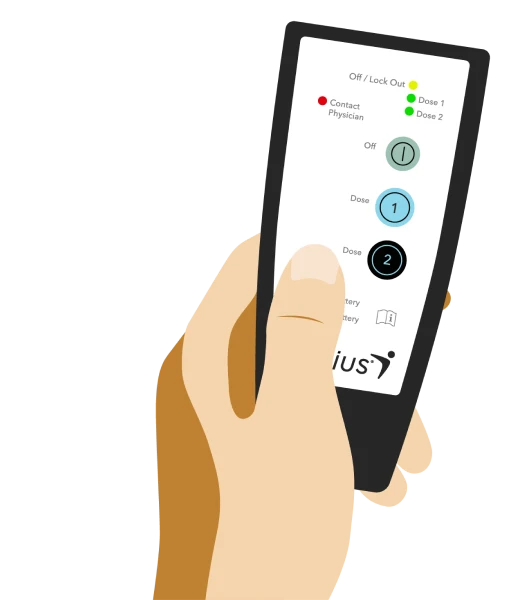
With the Altius patient controller, you can start treatments with the push of a button.
Powerful Relief. Proven Results.
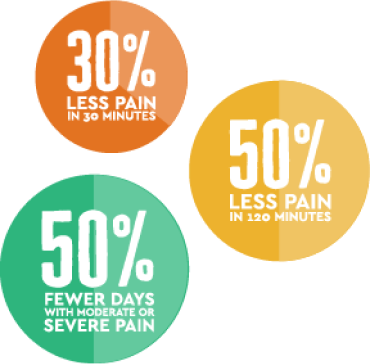
In a clinical study1 that followed patients through 12 months, patients treated with Altius reported:
Pain Reduction*
30% less pain in just 30 minutes
50% reduction in pain in 120 minutes
50% fewer days with moderate or severe pain

Opioid Reduction
81% of patients taking opioids decreased or eliminated opioid use

Quality of Life
45% improvement in quality of life
*Patients in the clinical study started therapy with Altius upon feeling pain. Patients averaged ~30% reduction in pain 30 minutes after starting a session and ~50% reduction in pain 120 minutes after starting a session. Days with pain was defined as pain greater than or equal to 4 on a pain intensity scale of 0 to 10.
Hear From An Altius Patient

Indications for Use: The Altius® Direct Electrical Nerve Stimulation System is indicated as an aid in the management of chronic intractable phantom and residual lower limb post-amputation pain in adult amputees.
Contraindications: The Altius system is contraindicated for patients who are: Unable to operate the system or Unsuitable for the Altius implant surgery.
Warnings/Precautions: Use as indicated and instructed. Diathermy should not be used on patients with the Altius System, or any of its components, either as a treatment for a medical condition or as part of a surgical procedure. Electromagnetic interference (EMI) is a field of energy generated by equipment found in the home, work, medical, or public environments that is strong enough to interfere with Altius system function. The electrical pulses from the Altius system may interact with the sensing operation from a cardiac device and could result in an inappropriate response of the cardiac device; physicians involved should discuss the possible interactions between the devices before surgery. Safety and Effectiveness of Altius System for pediatric use and for pregnant patients has not been established. Surgical complications and adverse events may be more frequent and severe in diabetic patients. Safety of MRI/NMRI with an implanted Altius system has not been evaluated. Patients implanted with the Altius System, or any of its components, should not be subject to MRI/NMRI. See Instructions for Use for detailed information regarding the procedure(s), indications, contraindications, warnings, precautions, and potential adverse events. For further information and to view full Instructions for Use, visit www.neurosmedical.com.
Although FDA has determined that the probable benefits outweigh the probable risks, there remains some uncertainty regarding the manufacturer’s human factors engineering (HFE) and usability engineering (UE) analysis and validation testing. As a condition of approval, FDA is requiring the manufacturer to provide an HFE/UE analysis and validation testing and recommending that this analysis and testing is designed using the FDA’s 2016 guidance document “Applying Human Factors and Usability Engineering to Medical Devices” (https://www.fda.gov/media/80481/download).
This information is for informational purposes only, is not medical advice or a diagnosis, and is not a substitute for professional medical advice. Consult with your doctor regarding your condition. Individual symptoms, circumstances, and results vary.
1. Kapural L, Kim B, Eidt J, et al. Long-term treatment of chronic post-amputation pain with bioelectric nerve block: 12-month results of the randomized, doubled-blinded, crossed-over QUEST study. Neuromodulation. 2024. 180 patients were enrolled in the clinical study. Individual patient outcomes may vary.
Rx only.