Give Your Patients power over pain
Altius is a first-of-its-kind device to help treat chronic, post-amputation pain in lower limb amputees.

The Altius® System
The Altius® Direct Electrical Nerve Stimulation System is the first device FDA-approved to specifically address chronic post-amputation pain. Altius is indicated as an aid in the management of chronic intractable phantom and residual lower limb post-amputation pain in adult amputees. It is a patient-controlled, on-demand system that uses Neuros’ patented technology to address the underlying cause of post-amputation pain by inhibiting pain signal transmission from damaged peripheral nerves to the central nervous system.
See how Altius Works
Intuitive, Outpatient Implantation
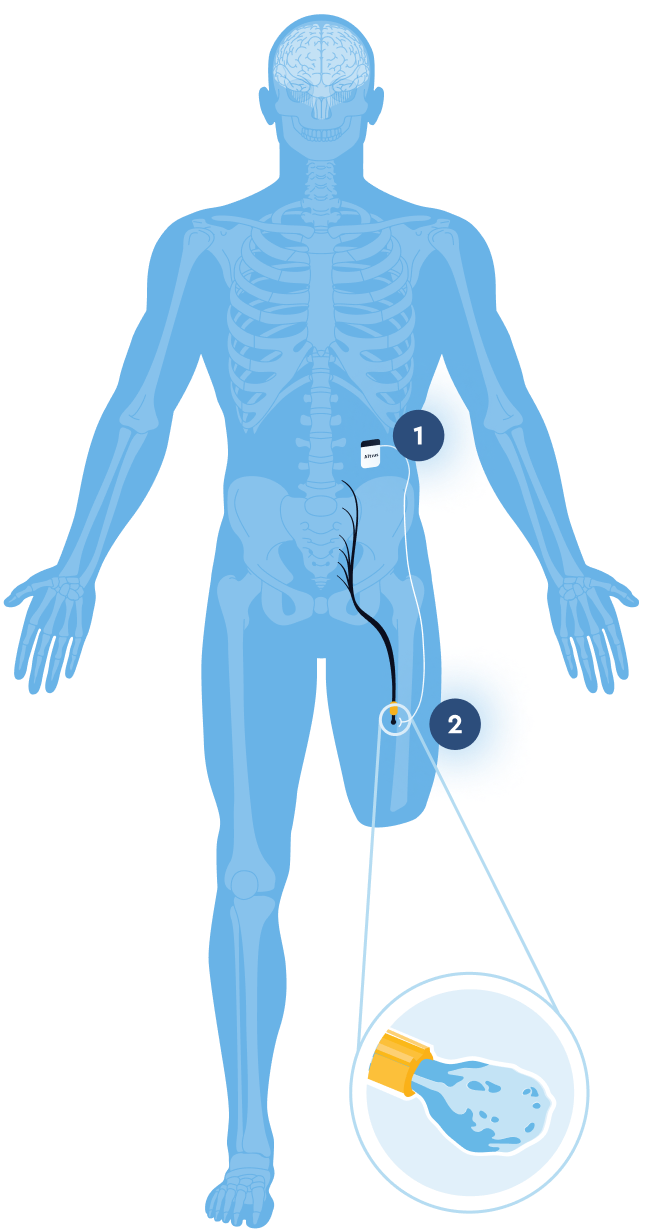
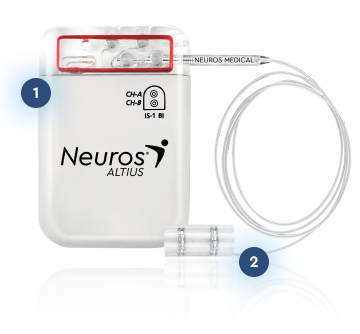
1. Rechargeable IPG
Implanted subcutaneously in the abdomen
2. Cuff Electrode
Placed locally on targeted nerve(s) near amputation site
On-Demand, Patient-Controlled
Pain Relief
PATIENT
CONTROLLER
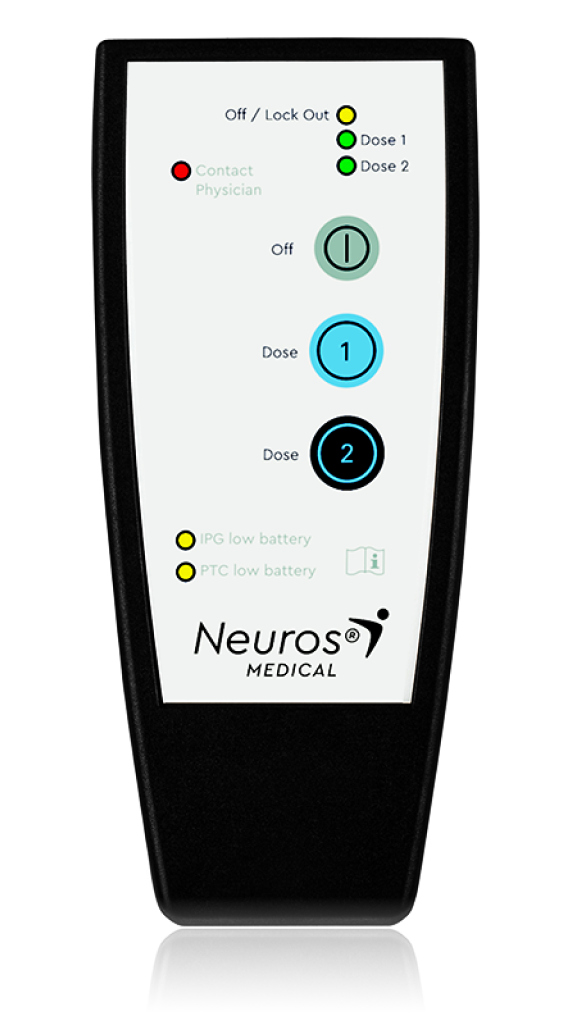
Rapid
on-demand pain relief
Easy
30-minute treatment sessions
BATTERY
CHARGER
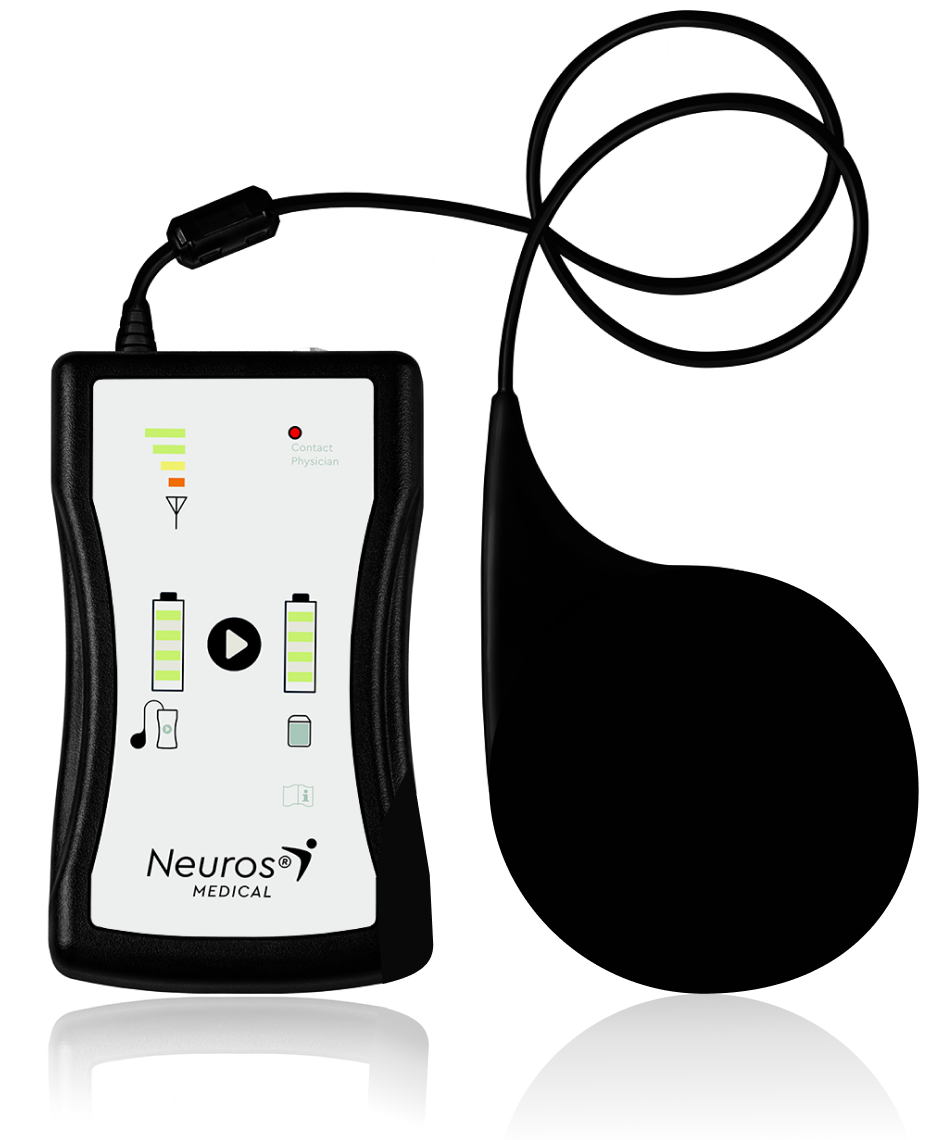
Convenient
charging
Straightforward
paddle placement on IPG
Significant relief. Proven results.
The Altius system was evaluated in the pivotal QUEST study: a multicenter, double-blinded, randomized controlled trial.1 The study enrolled 180 patients with a unilateral lower limb amputation across 35 sites in the US and followed patients for 12 months. QUEST is the largest, prospective RCT ever conducted for treatment of chronic post-amputation pain. In this study, treatment with Altius resulted in clinically significant and lasting pain reduction, decreased opioid use, and improved quality of life in amputees suffering with chronic post-amputation pain. See the results:
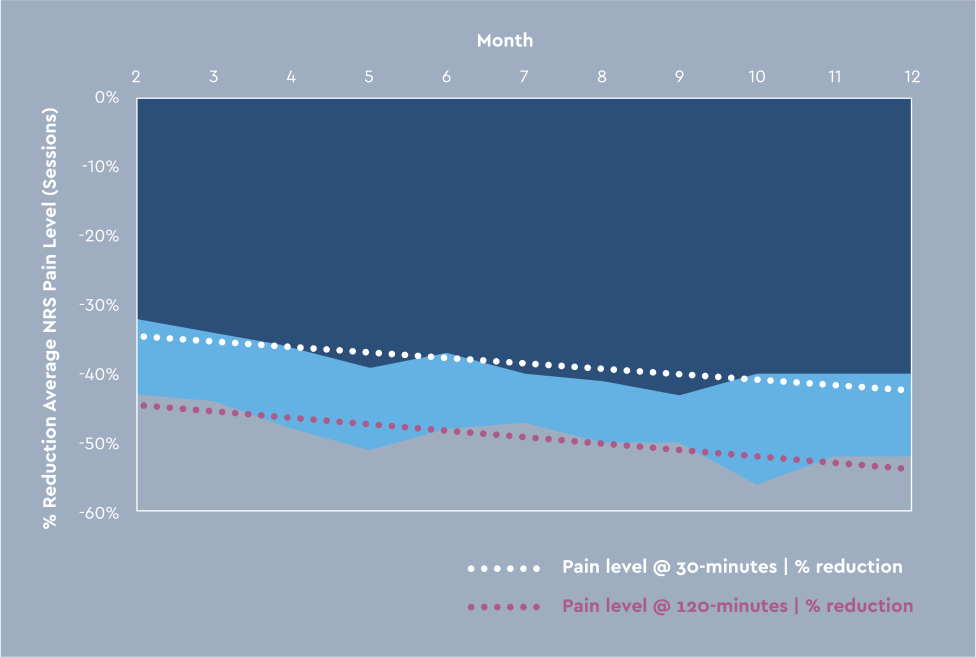
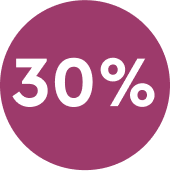
30% Pain reduction 30 minutes after initiating treatment.
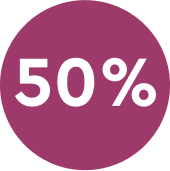
50% Pain reduction 120 minutes after initiating treatment.

Continued relief through 12 months with no observable therapy fatigue
Learn more about the QUEST results
Interested in Learning More About The Altius® System?
1. Kapural L, Kim B, Eidt J, et al. Long-term treatment of chronic post-amputation pain with bioelectric nerve block: 12-month results of the randomized, doubled-blinded, crossed-over QUEST study. Neuromodulation. 2024. FDA Trial NCT02221934.
Indications for Use: The Altius® Direct Electrical Nerve Stimulation System is indicated as an aid in the management of chronic intractable phantom and residual lower limb post-amputation pain in adult amputees.
Contraindications: The Altius System is contraindicated for patients who are: Unable to operate the system or Unsuitable for the Altius implant surgery.
Warnings/Precautions: Use as indicated and instructed. Diathermy should not be used on patients with the Altius System, or any of its components, either as a treatment for a medical condition or as part of a surgical procedure. Electromagnetic interference (EMI) is a field of energy generated by equipment found in the home, work, medical, or public environments that is strong enough to interfere with Altius System function. The electrical pulses from the Altius System may interact with the sensing operation from a cardiac device and could result in an inappropriate response of the cardiac device; physicians involved should discuss the possible interactions between the devices before surgery. Safety and effectiveness of the Altius System for pediatric use and for pregnant patients has not been established. Surgical complications and adverse events may be more frequent and severe in diabetic patients. Safety of MRI/NMRI with an implanted Altius System has not been evaluated. Patients implanted with the Altius System, or any of its components, should not be subject to MRI/NMRI. See Instructions for Use for detailed information regarding the procedure(s), indications, contraindications, warnings, precautions, and potential adverse events before performing the Altius procedure. For more information and to view full Instructions for Use, visit www.neurosmedical.com.
Although FDA has determined that the probable benefits outweigh the probable risks, there remains some uncertainty regarding the manufacturer’s human factors engineering (HFE) and usability engineering (UE) analysis and validation testing. As a condition of approval, FDA is requiring the manufacturer to provide an HFE/UE analysis and validation testing and recommending that this analysis and testing is designed using the FDA’s 2016 guidance document “Applying Human Factors and Usability Engineering to Medical Devices” (https://www.fda.gov/media/80481/download).
This information is provided for educational or informational purposes only and should not be considered medical advice or a directive for clinical practice. This information does not guide specific patient treatment decisions. Neuros Medical does not recommend or endorse any particular course of treatment or medical choice. Healthcare professionals are advised to base their clinical decisions on their professional judgment and patient-specific factors.
Rx Only.